Comments on the Oregon Coast Hatchery DEIS
Purpose of the Oregon Costal Hatchery Program and HGMPs
The DEIS on Hatchery Genetic Management Plans (HGMPs) is a standalone proposal unrelated to genetic and ecological impacts hatcheries have on wild salmonids outside the hatchery fence. HGMPs are treated in isolation from harvest impacts on wild salmonids and associated fisheries on spawner escapement and return of marine derived nutrients to watersheds from carcasses of naturally spawning wild salmon. Smith (2000) in the NMFS template for HGMPs states: “When “take” of a listed species is expected in the hatchery operation, (emphasis added) the ESA requires that a numerical estimate be quantified as best as possible.” The HGMP template is confined to hatchery operations and to impacts on listed species. Impacts outside the operation of the hatchery are not included in the HGMP and on the Oregon coast the only protected species is coho salmon. So it is unclear how the proposed HGMPs in the preferred alternative will address impacts on non-listed wild salmonid species and listed coho outside the hatchery in Nature.
At the time the HGMP template was developed coho salmon on the Oregon coast were not listed but Smith (2000) states: “Hatchery programs located outside of the Columbia Basin that are not involved in the artificial propagation or research of ESA-listed salmonid populations therefore do not have to complete Sections 8 (“Mating”), 9 (“Incubation and Rearing”) and 12 (“Research”) of the HGMP template [although managers are encouraged to do so].” Therefore the HGMPs are not required to address non-listed species. Because there is no requirement for ODFW and NMFS to follow HGMP protocol for steelhead, chum, and chinook, there is no assurance that HGMPs will be binding for other species in the DEIS proposed alternative.
Even for coho salmon Smith (2000) says: “NMFS will evaluate each HGMP for ESA…that are designed to minimize take and promote conservation of the listed species that may be affected by the hatchery program. The completed HGMP must therefore indicate anticipated take levels, and specific management measures that minimize take of listed species and protect listed ESUs.” (emphasis added)
Smith (2000) also notes that “G) Under the broad definition of ESA, “take” of listed species will include hatchery activities that lead to harassment, behavioral modification, capture, handling, tagging, bio-sampling, rearing, release, competition, predation, disease transfer, adverse genetic effects, injury, or mortality of listed fish. When “take” of a listed species is expected in the hatchery operation, the ESA requires that a numerical estimate be quantified as best as possible.”
Unless “minimize take” means zero NMFS and ODFW will have to explain what minimize means in measurable terms and the monitoring program that will be used to verify it. But would this be required for take of all species not just listed coho salmon?
The track record for HGMPs for Sandy and McKenzie rivers represents that both NMFS and ODFW disregarded the impact of their hatchery programs on wild salmonids. They were sued by third parties for negligence and the court agreed, modifying releases of hatchery fish.
In the DEIS, NMFS says “All of the hatchery programs are managed solely for fishery harvest opportunity.” (Page 3-14) The NMFS and ODFW are committed to increase hatchery product for commercial fisheries and in doing so increase the risk to wild salmonids in coastal rivers including coho salmon.
NMFS also confirms a hatchery bias when it states as a fact that “…hatcheries can be managed in a manner that conserves and recovers salmon and steelhead listed under the ESA.” (NMFS 2000). This conclusion by NMFS ignores the weight of peer reviewed scientific studies that document hatchery salmonids have lower survival, are less diverse, and have lower reproductive success, than wild salmonids and cause genetic and ecological impacts when hatchery fish spawn naturally in rivers.
Review of hatchery programs by the HSRG states: “Hatcheries are by their very nature a compromise—a balancing of benefits and risks to the target population, other populations, and the natural and human environment they affect” (Paquet et al. 2011). It is obvious that NMFS and ODFW do not agree given their operation of hatcheries in the Columbia and Willamette rivers that failed federal court review. There is now another third party lawsuit on a collection of lower Columbia River hatcheries regarding excessive releases of hatchery chinook and the straying problem they create. At each turn, when the federal court reviews HGMPs the NMFS and ODFW fail to be in compliance with recommendations by the HSRG and criteria proposed by NMFS in Smith (2000).
Paquet et al (2011) also cautioned the NMFS and ODFW among other fishery agencies to pay attention to the latest scientific review of hatcheries: “Hatchery strays on the spawning grounds pose a risk. For conservation programs the demographic benefits of hatchery fish on the spawning grounds must outweigh the risks. HSRG conservation solutions that shift spawning dynamics from conditions of high hatchery influence to conditions of low hatchery influence are designed to provide the greatest benefits for wild stocks.” When NFS sued ODFW and NMFS on a seventy percent stray rate for hatchery chinook in Sandy River it was opposed until the federal court agreed that the government was not in compliance with the HGMP and cut the releases of hatchery fish.
“The fitness assumptions (Paquet et al. 2011) used in AHA result in a 50% loss of natural productivity when the contribution of hatchery fish is high (pHOS > 10%) for several generations. On the other hand, when the hatchery contribution is low (e.g., pHOS < 5%) over several generations, the natural productivity approaches that of a locally adapted natural population.” By following this scientific direction the HSRG (Paquet 2011) admits that hatchery fish impact the fitness of natural populations even at recommended low stray rates.
The ISAB (2016) recommends “additional empirical assessments are needed to verify their adequacy for protecting the fitness of natural populations.” In their Critical Uncertainty report to the Power Planning and Conservation Council there is uncertainty “about the genetic or epigenetic changes that occur in cultured populations, and the impacts of such changes on the fitness of natural populations.”
Hatcheries are a “compromise “ that affects the reproductive success of wild salmonids even when stray rates are less than 5%, causing genetic and ecological impacts on wild salmonid populations exposed to hatchery strays, competition, and predator attraction.
Most wild native salmonid populations have declined to less than 5% or their historic abundance. The impact of hatchery strays on vulnerable small wild populations can be even more compromising than the HSRG recommendations point out.
A panel of scientists convened by NMFS concluded that they “found no genetic justification for allowing gene flow from non-native fish (hatchery strays) at levels as high as 5%” (Grant 1997)
“Genetic risks increase substantially when the proportion of the adult population that is hatchery fish increases over 5% “(Lynch and O'Hely 2001; Ford 2002).”
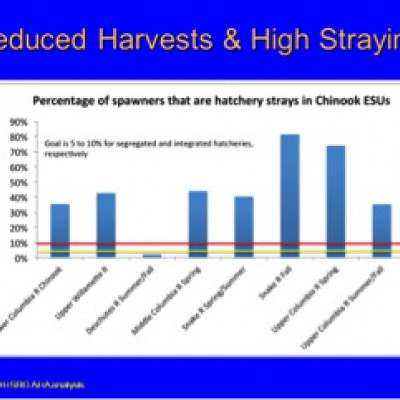
The Columbia River is the grand experiment on using hatcheries to support fisheries and recover 109 wild salmonid populations at risk of extinction. As indicated the graphic the failure of state and federal fishery agencies to comply with 5-10 percent limit on pHOS or stray hatchery fish. The only watershed in compliance is one that has no associated hatchery program. The Columbia River salmon management and recovery program is a failure, but we know that because of the funding available to evaluate the impact of hatchery programs on watersheds and populations. On the Oregon coastal watersheds the funding and independent scientific evaluation is not available, leaving the state and federal fishery management agencies free of accountability for their policy and criteria to protect wild salmonids. For example in the ODFW final Multi-Species Management Plan (CMP 2014) efforts to assess impacts “if feasible” and monitoring will “seek to address stray rates” (page 204) suggests that the NMFS DEIS preferred alternative lacks assurance that pHOS criteria will actually be applied.
Even though hatcheries are considered a “compromise” (Paquet et al. 2011) and scientific evaluation clearly documents genetic and ecological changes in hatchery fish making them vectors in the effort to recover wild salmonids (Araki et al. 2008; Araki et al. 2009; Araki and Schmid 2010; Christie 2016, Chilcote 2013 ) the fishery agencies are immune to peer reviewed science. They continue to ignore it in their focus on producing hatchery fish for harvest and using inadequate mitigation measures. A recent review of the fish and wildlife program by the ISAB\ISRP (2016) recommended fishery management “focus on sustainability with strategies to protect diversity and resilience, and to build adaptability.”
It is not clear that the NMFS Science Center and the Protected Species Program are involved with Sustainable Fisheries Program in establishing criteria to protect listed coho and non-listed species on Oregon coastal streams and HGMPs. The result is hatcheries and associated fisheries do not have comprehensive internal review that would help to refocus management suggested by the ISAB\ISRP (2016). As it now stands the Columbia River salmon recovery program for wild salmonids is compromised by harvest and hatchery policy actions. Therefore it is unreasonable to expect correction of this problem by adopting the proposed alternative in the NMFS DEIS for Oregon coastal coho and other wild salmonids.
The DEIS describes numerous negative impacts of artificial propagation on wild, native salmonids. Salmonids are a public resource and are to be managed for the public interest by state and federal government agencies. Negative impacts of hatcheries primarily result from serving the economic interests of commercial and sport fisheries at the expense of and risk to wild salmonid productivity, diversity, distribution, and abundance. This is a historical problem associated with salmonid management of over 100 years. The DEIS does not discuss this historic issue or propose changes in institutional organization to establish an effective conservation program to protect wild salmonids and listed coho salmon. The DEIS has assumed that the status quo is the objective of fishery management and that hatcheries and associated fisheries are to continue along the historic path. Changes in hatchery management through HGMPs are unproven as an effective conservation measure yet are assumed to contribute to a trend toward conservation.
Since hatcheries and associated fisheries represent a known risk to wild native salmonids the logical outcome of the DEIS for threatened coho salmon and for wild steelhead, chinook, chum and cutthroat is continued depletion and extinction in the Oregon coastal rivers. The public interest will not be served even though the public has periodically taken legal action though initiative petitions and the courts to change the institutional management of salmonids.
The only alternative that would improve conservation is to manage salmonids on a river specific basis by species and race. This includes a spawner abundance objective for salmonids in each watershed, regulation of fisheries to achieve that escapement objective, and manage hatcheries to not interfere using measurable conservation criteria applied to each watershed salmonid population.
The preferred alternative in the DEIS would only continue the aggregated management of salmon as if locally adapted breeding populations by watershed were not important. This institutional impediment to conservation means increased risk to wild salmonids is the only logical outcome.
Habitats that sustain the freshwater and estuary productivity of wild salmonids have not been adequately addressed by state and federal land and water managers. Rather than promote habitat protection the fishery managers use habitat issues to justify hatchery production and argue for more hatchery funding to support fisheries and in doing so to grow agency budgets. Fishery agencies have also opposed protection and conservation of wild salmonids. The fight to list coho salmon as a federal protected species is a good example of that resistance by fishery institutions and state and federal government to maintain the status quo, using public funded hatchery programs to replace natural production of wild salmonids in Oregon coastal watersheds.
Knudsen (2000) asked an important question. “What would production be like if escapements were of historic magnitude, i.e., about ten or more times greater than today’s? Since it is unlikely that freshwater habitat quality has been reduced 90% coastwide, as escapements have, ideal contemporary escapement goals probably lie somewhere between those observed today and ten times as much.”
SPECIFIC REVIEW OF THE DEIS-NEPA
Evaluation of Alternatives
The alternatives provided are not adequate to evaluate the effect of the hatchery HGMPs on threatened coho and other species. For example, alternatives 1 (No Action) proposes to adopt the HGMPs proposed by ODFW without evaluation regarding their impact on listed and non-listed salmonids. This would allow ODFW to continue operating hatcheries as they have been without any evaluation. Alternative 2 (Preferred) is equal in all respects to alternative 1 because it would allow HGMPs to function as they have been by ODFW. In other words these two alternatives (1) and (2) are the same and lack NMFS evaluation for impacts on the environment. In the DEIS the NMFS has abdicated its responsibility to approve, modify or deny the HGMPs and show evidence that this responsibility has been carried out based on scientific evidence. Consequently the public is provided no information to compare and therefore respond to these two alternatives since they are the same in their effect on the environment.
Alternative 3 (stopping all hatchery releases) is not recommended by NMFS because it would harm commercial and recreational fisheries. Since the HGMPs and associated hatcheries are the primary concern of ODFW and the exclusive concern of the Sustainable Fisheries Program of NMFS administrative organization, it is logical that the primary concern would be fisheries rather than species protection. A balanced evaluation that would also include the Protected (Species) Resources Program of NMFS organization and science center review is needed to evaluate the effect on ESA threatened coho salmon and other natural salmonid populations by closing some or all hatcheries. It is through a comprehensive evaluation by NMFS involving both sustainable fisheries, science, and species protection programs that an adequate assessment of alternative 3 is possible. Alternative 3 reveals the bias of NMFS (2000) to perpetuate the status quo management of salmonids on the Oregon coast to provide product for the market economy rather than balancing that with species protection. “NMFS believes hatcheries can be managed in a manner that conserves and recovers salmon and steelhead listed under the ESA” (NMFS 2000). This belief is not supported by the best available science (Chilcote et al 2013; Araki 2009; Araki et al. 2008; Araki and Schmidt 2010; Buhle 2009; Kostow et al. 2003; McLean et al. 2004; Naish et al 2008; Nickelson 2003; Oosterhout et al 2005; Reisenbitcher and McIntyre 1977; Reisenbichler and Rubin 1999; Seamons 2012; Scheuerell et al 2015;Theriault 2011; Utter 1998. Christie et al. 2014, 2016).
Consequently, alternative 3 fails to evaluate the benefits of closing hatcheries for both fisheries and conservation. Alternative 3 is cast in an unfavorable light by the DEIS because it would damage commercial interests, thus perpetuating the 150 year record of management for salmonids that has caused severe decline in abundance and increased risk to wild salmonids in the Northwest.
Alternative 4 (Reduce hatchery production by 50%) is not permitted but can be evaluated by NMFS. In other words, NMFS is constrained by its own rules to make adjustments in hatchery program releases to protect threatened species and other salmonids under 4(d) regulations. Consequently, this alternative is not actually real and cannot be selected due to NMFS lack of authority and its primary concern about impacts to industrial fisheries (commercial and sport).
Based on our review of alternatives 1-4 we conclude that the draft EIS is substantially flawed and cannot meet the requirements of the National Environmental Policy Act.
Issues that are not adequately addressed by HGMPs in the NEPA DEIS
In reviewing the HGMPs where the focus is on hatchery operations to reduce or eliminate take under rules of the ESA, it is unclear how take related to hatchery fish impacts on the environment once they are released from the hatchery. It is my understanding that the HGMPs must also address impacts related to fish once released.
On the Oregon coast hatcheries and HGMPs are managed to supply fish for harvest. Hatcheries and harvest are two sides of the same coin, but the hatchery fish once released from the captive environment into natural streams and related harvest have an impact on listed, candidate and non-listed species. These impacts relate to ecological and genetic impacts, nutrient enrichment of streams by naturally spawning salmonids, stray rates, residual hatchery fish that do not migrate, and habitat management.
Therefore I would like to know exactly how the HGMPs are to effectively protect candidate, listed and non- listed salmonids following release from hatcheries. This is not adequately addressed in the EIS DEIS document. I address each of those problems below.
pHOS
(Page 4-24) As long as pHOS continues to be low (<10 percent) population wide, it is expected there will be reduced productivity from naturally-spawning hatchery fish interbreeding with natural-origin coho salmon. However, there are 26 other independent populations where no hatchery coho salmon programs exist. In total, only four out of a total of 69 independent and dependent coho populations would be affected genetically by the hatchery coho salmon programs released into these populations.
Comment:
NMFS and ODFW rely on the unevaluated theory of pHOS. The ISAB said, “This is an interim standard that should be reviewed and updated as better information becomes available. How the HSRG management guidelines, or variations of them, affect the reproductive fitness of natural populations has not been assessed.” (ISAB\ISRP 2016)
There is no substantiated evidence that 10% pHOS or less actually protects ESA threatened Oregon Coastal Coho Salmon and other wild salmonids from genetic and ecological impacts from hatchery programs. Therefore, it is scientifically inappropriate to rely on any pHOS level as protective of threatened species and other wild naturally reproducing species such as steelhead, chinook, chum and cutthroat trout.
Furthermore, NMFS and ODFW assume, inappropriately, that the biological effects of hatchery releases are confined to the watershed in which they are released. Therefore not all hatchery strays are evaluated in the DEIS. There are intentional strays when fish are transferred among watersheds and unintentional strays that do not return to the hatchery and spawn in the release stream or adjacent streams. All naturally spawning stray hatchery fish can have genetic and ecological impacts on wild conspecifics within hatchery dominated streams and streams that do not have hatchery releases. Complete evaluation of strays and their origin should be required in the DEIS to determine their impact on naturally spawning wild salmonids. Following this evaluation effective measures to reduce hatchery strays would be required by the DEIS for all species to prevent non-listed salmonids from potential ESA listing, and promote recovery of listed coho and steelhead a candidate species.
Hatchery Strays
Comment:
Stay hatchery fish of all species released into a watershed produce strays that contribute genetic and ecological impacts in streams with no releases and in areas of a watershed that hatchery salmonids stray into. Since this stray effect is not addressed nor quantified, the DEIS and NEPA is not sufficient.
The DEIS states: “Along the Oregon Coast, most natural populations currently have low percentage of hatchery fish on the spawning grounds (e.g., 0 to 10 percent). Therefore, managers are able to evaluate the true status of the natural population because hatchery influence is relatively minor.” (Page 3-15) The operative word in this quote is “most” which suggests stray rates are higher for some species such as steelhead and fall chinook.
“Recipient population straying estimates for winter steelhead have been limited. One exception was Schroeder et al. (2001), who estimated that hatchery winter steelhead strays made up 4–43 % of several winter steelhead populations in coastal Oregon rivers.” (Keefer and Caudill 2013)
Hatchery salmonids stray more because “…reduced stimuli in hatchery fish have been associated with lower hormone levels (Dittman and Quinn 1996; McCormick et al. 2003) as well as lower olfactory activity and reduced brain development (Marchetti and Nevitt 2003) compared to wild fish. For these reasons—and probably others—hatchery fish are widely believed to have reduced imprinting relative to wild fish.” (Keefer and Caudill 2013)
“The weight of evidence indicates that human activities- including many common fisheries, river management, and propagation practices- increase anadromous salmonid straying rates. There are certainly no universally ‘appropriate’ straying rates that can be used as management targets.” (Keefer and Caudill 2013) This conclusion supports the conclusion of the ISAB\ISRP (2016) regarding pHOS of 10% for hatchery strays is an unverified theory.
The DEIS states: “ODFW has recently completed their Coastal Multi-Species Conservation and Management Plan (CMP) for the Oregon Coast Region. The current hatchery programs reflect the decisions under this management plan. The Best Management Practices (BMPs) used by ODFW for hatchery management would also continue as described in the submitted HGMPs.”
While the NMFS DEIS has confidence in the ODFW’s CMP this plan confesses hatchery salmonids do stray but ODFW has not “assessed” stray rates to determine “exact rates.” These facts would undermine the assurance in the DEIS that HGMPs would function to protect wild salmonids in coastal rivers and that pHOS criteria are implemented as required. In fact funding may limit these actions as ODFW states in the CMP: “New management and monitoring efforts will require new funding. All new actions and projects will be contingent on available funding and staff.” Even though NMFS knows that ODFW has initiated an advisory group to assess funding options to support management commitments, the DEIS represents actions that would take place in the preferred alternative that lack funding.
“Hatchery Fish have the potential to cause either genetic or ecological…impacts on any population with which they spatially and temporally overlap (Araki et al. 2008; Buhle et al. 2009; Chilcote et al. 2013). However, specific effects of coastal hatchery programs have not been systematically assessed. Information exists that hatchery winter steelhead stray onto spawning grounds in some locations, although exact rates are difficult to assess, and this information has not yet been analyzed.” (ODFW 2014 Coastal Multi-species Conservation and Management Plan)
Even though ODFW admits in the CMP that “exact stray rates” have not been determined information in Table A-III: 4. (page 175) identifies “Stray rate targets for hatchery fish on natural spawning grounds (pHOS).” Even though some of those targets are excessive, they are still unconfirmed management targets. The NMFS DEIS is based on facts not in evidence.
There was no independent scientific review other than that provided by NFS that pointed out problems with this management plan (CMP) as a conservation plan for non-listed steelhead, chinook, chum, and cutthroat trout. Coho salmon were not included in the CMP evaluation.
Marine Derived Nutrients from Salmonids
(Page 3-19) “The proposed action includes the benefit of marine-derived nutrients into the freshwater environment from hatchery fish returns.
(Page 3-19) “More than 80 percent of the marine-derived nutrients available to the freshwater environment have come from natural-origin salmon and steelhead carcasses in the project area.”
(Page 3-19) “In 2012, more than 16,336 hatchery fish carcasses were outplanted within the Oregon Coast Region (ODFW 2013d).”
“Hatchery fish that are not harvested or collected at hatchery facilities can spawn in the wild and contribute marine derived nutrients to the environment.
Comment:
While the DEIS asserts that natural spawning hatchery fish have a value by depositing marine derived nutrients into coastal streams, benefiting productivity of wild salmonids, it also states a number of associated risks such as “genetic introgression, increased competition, predation from hatchery fish, transfer of pathogens, discharging effluent, masking of natural population status.”
The DEIS does not discuss the relative value of naturally spawning hatchery fish nutrient benefit and associated risks to wild salmonids. Since “more than 80 percent of marine derived nutrients come from naturally spawning wild salmonids,” the contribution hatchery salmonids is relatively minor compared to the risks. The DEIS also fails to discuss the greater benefits of naturally spawning wild salmonids that range from gravel cleaning, riparian vegetation growth, stream productivity, seasonal food resources that benefit wild life, and terrestrial insect production.
The DEIS states that only those fish not harvested provide a nutrient benefit to streams. Nutrient enrichment of streams from naturally spawning hatchery fish is driven by harvest not by any biological objective for nutrient enrichment for each stream. The DEIS does not establish nutrient enrichment objective by river. The risks associated with harvest and hatchery impacts on wild salmonids that provide the greater nutrient contribution to streams are not addressed.
However, NMFS (2008) did address nutrient enrichment of streams from naturally spawning coho salmon. “Coho salmon are an important contributor to stream nutrient supply, and Bilby et al. (2001) estimated the density of coho salmon carcasses that would maximize nutritional benefit to juvenile coho salmon at approximately 120 carcasses per km of spawning habitat. Given about 6,900 km (4,300 miles) of spawning habitat in the range of the ESU, this translates to an escapement of about 830,000 spawners to saturate the stream nutrient supply.”
Bilby (2001) also said: “Restoration of freshwater habitat in the region will require that the role Pacific salmon play in maintaining the health of freshwater habitats be considered in establishing harvest levels and escapement goals for these fishes.”
Establishing escapement goals for wild salmonids which provide the highest proportion of marine derived nutrients to coastal streams is needed for all species due to their “The important contribution these fishes make to the productivity of the habitats where they spawn and rear.” (Bilby 2001).
In the proposed alternative the DEIS attempts to make a positive case for marine derived nutrient delivery from naturally spawning hatchery fish and from hatchery carcasses dumped into streams. However, the risks associated with naturally spawning hatchery fish on wild salmonids that provide over 80 percent of these nutrients are ignored. The DEIS does not set an escapement objective for wild fish into each coastal stream to supply needed nutrients from naturally spawning salmonids. Relying on the HGMPs to supply nutrients to streams increases risk to wild salmonids and therefore is incompetent.
A nutrient enrichment target from naturally spawning salmonids is needed to improve productivity for listed coho salmon, candidate steelhead, and non-listed salmonids. Harvest would be managed to deliver nutrient targets by watersheds.
Escapement
Escapement in the DEIS is defined: “Adult salmon and steelhead that survive fisheries and natural mortality, and return to spawn. Run size: The number of adult salmon or steelhead (i.e., harvest plus escapement) returning to their natal areas.
Both definitions treat escapement generally without reference to watersheds or spawner abundance goals by locally adapted population. These definitions are derived from a management framework based on species abundance and aggregated harvest. The DEIS definition of escapement is inconsistent with conservation and recovery of depleted wild salmonids established by NMFS (McElhany et al. 2000) for viable salmon populations (VSP) using criteria for abundance, productivity, spatial structure, and diversity of natural origin (wild) salmonids. These criteria are used to evaluate the biological status of independent populations by watershed. Wild salmonids are at greater risk when there is a conflict between management definitions use by state and federal fishery agencies. Management of salmonids using VSP criteria is necessary to recover depleted populations of salmonid by watershed and to prevent low risk populations becoming depleted and protected through the Endangered Species Act. So the definition for biological escapement management must be consistent with VSP criteria for natural origin (wild) salmonids by watershed including protected coho salmon.
Coho Salmon Example
The hatchery reforms due to ESA-listing in the late 1990s which substantially curtailed hatchery releases of coho salmon in the Oregon Coast ESU substantially reduced the impacts of hatchery fish on natural-origin coho salmon populations. Buhle et al. (2009) evaluated the reduction of hatchery coho salmon releases in the ESU and concluded there was an approximately 20 percent increase in productivity from the reductions in pHOS. Productivity increased by another seven percent from the reductions in ecological impacts associated with hatchery coho salmon smolt releases interacting with natural-origin coho salmon. Therefore, approximately 27 percent of the improvements to the status of coho salmon in the Oregon Coast ESU could be attributed to the hatchery reform actions taken since ESA listing (Buhle et al. 2009). (Page 3-21)
In evaluating salmonid escapement management Knudsen (2000) said: “Over 44% of 9,430 identified populations or 2,925 populations for which there is information are not monitored for escapements. It remains obvious that both escapement data collection methods and the programs to collect high quality escapement information are deficient. To achieve overall sustainability, it is always preferable to identify likely spawning aggregations as the smallest unit…then to develop management plans for protecting abundance and genetic diversity based on that knowledge.” The smallest unit would be defined at the watershed scale.
Knudsen (2000) recommends sound management of salmonids would “…ensure that escapements are sufficient to perpetuate maximum biomass production and biodiversity. We cannot count on repairing only one damaged aspect of salmon runs (e.g. degraded habitat) to fix the problem, but must work on all fronts simultaneously. Ultimately, though, both productivity and biodiversity depend on sufficient escapement of spawners to fully utilize the available freshwater habitat, fertilize the systems with carcasses, and optimize genetic diversity.”
Harvest has to be regulated to return spawner abundance (escapement) by species and race to each stream. This would support nutrient enrichment, genetic diversity, productivity and spatial structure for each species in order to achieve recovery coho salmon, candidate steelhead and prevent depletion of non-listed salmonids. Each stream and its populations of native, wild salmonids becomes a management unit based on measurable criteria that define its health and productivity.
Residual hatchery smolts become resident competitors
“The potential for hatchery fish to negatively impact wild fish has been identified as a concern for dwindling stocks of naturally produced anadromous salmonids in the Pacific Northwest. (McMichael 1999)
“…when hatchery steelhead become residuals, thus increasing local densities of salmonids for extended periods, the growth of sympatric wild rainbow trout growth is likely to decrease. A reduction in size, due to slower growth during the summer, could decrease overwinter survival , resulting in decreased population size. (McMichael 1997)
“Hatchery steelhead displaced wild O. mykiss in 79% of the contests observed between these groups. Our results indicate that the behavior of hatchery steelhead can pose risks to preexisting wild O. mykiss where the two interact. Strategies to minimize undesirable risks associated with behavior of released hatchery steelhead should be addressed if protection and restoration of wild O. mykiss stocks is the management goal.” (McMichael et al. 1999)
Levels of residualism for 130,000 to 200,000 hatchery steelhead smolts using differing steelhead smolt residualism levels found in the scientific literature
Residualism Rates Resulting Residuals Resulting Residuals In Differing Studies From 130,000 Smolts From 200,000 Smolts
Werlen 2003 * @ 1.6% 2,080 3,200
Keogh River
Viola/Schuck 1991 @ 17.7% 23,010 35,040
Tucannon River
Viola/Schuck 1992 @ 10.3% 13,390 20,600
Tucannon River
Viola/Schuck 1993 ** @ 3.1% 4,030 6,200
Tucannon River
Tipping et al. 1995 ***
1991 @ 26.1% 33,930 52,200
1992 @ 13.8% 17,940 27,600
1993 @ 19.6% 25,480 39,200
Snow Creek
WDG 1966 ****
(from Royal 1972) @ 44% 57,200 88,000
@ 35% 45,500 70,000
Elochoman River
Ward/Slaney 1990 @ 42% 54,600 84,000
@ 47% 61,100 94,000
Keogh River
McMichael et al. 2000 ***** @ 26% 33,800 52,000
@ 39% 50,700 78,000
Teanaway River
* Smolts acclimated in netpens in lake in upper watershed and then released in the lower Keogh at mean length of 180mm. Smolts above 200mm considered problematic as precocious males. Broodstock was wild.
** Smolts acclimated in lake, volitional emigration; release time 4/19-5/3, smolts released weighed 10.4 fish/kg. Broodstock was Lyons Ferry Hatchery Stock.
*** Smolts transported from South Tacoma Hatchery to release site 4.7 km upstream of fish trap. Residuals identified as primarily smolts released below 190mm. Smolts above 200mm were not found problematic, although the Methods section indicates precociously mature smolts were selected out prior to release which in many ways invalidated the experiment which was not discussed. Nevertheless, residualism remained comparatively high. Broodstock was Chambers Creek Hatchery Stock.
**** Smolts were reared at hatchery on Beaver Creek at 7 fish per pound and released on 4/15. Outmigrating fish were then counted at trap one mile downstream. Broodstock was Chambers Creek origin.
***** Smolts were transported from WDFW’s Yakima Hatchery and released into Jungle Creek trying to mimic volitional releases from an acclimation pond over a 10 day period. The study occurred from 1991-94 with varied levels of residualism and precocious males each year. Smolts released were from 179-201mm. Broodstock from Yakima Hatchery.
-------------
Residual hatchery steelhead not only compete with wild candidate steelhead they impose ecological impact on other species such as listed coho salmon, chinook, chum and cutthroat trout. These ecological impacts include competition for available food resources, rearing space including overwinter rearing space critical to coho salmon survival, predation, and predator attraction and interbreeding with adult female steelhead.
The DEIS does not include measurable criteria in the HGMPs that decrease these ecological impacts on protected coho, candidate steelhead, and non-listed salmonids, therefore the HGMPs are deficient and should not be approved by NMFS until the problem is resolved.
Economic Evaluation
“Given the increasing body of science showing deleterious impacts of hatchery production on wild stocks, it is only reasonable that natural replacement scenarios be included (NMFS 2016) and costs to replace wild salmonids with hatchery fish rather than invest in habitat and fishery management based on escapement and productivity for wild salmonids. It is possible to provide a more durable solution for salmon decline by investing public funds in natural production and maintain fishery opportunities (Ogston et al. 2015).
The DEIS relies on erroneous benefit-cost analysis (Radtke et al. 2016) because it does not account for construction and maintenance cost of hatcheries headquarter administration, long term capital costs, fishery management costs related to the harvest of hatchery fish should be included in the analysis (Carter et al 2010) By leaving out those cost factors in the benefit cost analysis, the benefits are overstated by a factor of two (Radtke et al. 2016).
“NMFS should sponsor their own net economic valuation analysis to be used in the DEIS that includes all policy-related hatchery benefits and costs. The benefit side should account for not only direct fishery costs, but also…conservation benefits, plus…negatives of hatchery production, such as diminished natural production due to weakened genetics, crowding, predator concentration.” (Radtke et al. 2016) Additional accounting can and should be provided for diminished nutrient return to watersheds from carcasses of naturally spawning salmon, and reduced escapement of wild salmonids that support genetic, life history, and ecological diversity for wild populations as well as a source of fish for artificial propagation.
The NEPA DEIS claims that alternatives would provide production levels that would result in a small fraction of the total harvestable salmon and steelhead in the analysis area, and would provide a small fraction of the overall economic benefits derived from harvest. This means that the risk to threatened coho salmon and other species of wild salmonids would be increased by the preferred alternative yet provides a minor economic benefit to the target user groups (page 5-26).
Economic evaluation is often manipulated by fishery agencies to put a shine on the artificial production program by focusing attention on the benefits and ignoring the costs to provide those benefits. Each hatchery program should provide a cost evaluation that includes the cost to provide a harvested salmonid as the IEAB (2002) provided. Since hatcheries are public funded, it is only reasonable to provide the public with a real evaluation of that public investment by the fishery agencies. This cost, of course, would also be expanded to include the impact of hatchery production and associated harvest in terms of costs related to their impact on wild salmonids including the investment in salmon habitat restoration. Hatchery salmon waste habitat investments in habitat because of their reduced fitness and impacts on natural populations. Until there is a real economic evaluation of artificial production the public will be manipulated by fishery agencies to believe that benefits are real. The DEIS is misleading and incomplete until a full economic evaluation of all costs are provided for public review.
Minimize Impacts
The term “minimize” is a popular term in fisheries management. It is primarily used when describing risks of hatcheries, harvest, and mitigation affecting wild salmonids. Unless minimize means zero impact it is necessary to explain the monitoring program that is in place and explain what will be done about anticipated impacts. The DEIS does not explain how impacts to wild salmonids are to be minimized and resolved. Some examples are provided below:
“This plan resulted in many changes to hatchery management along the Oregon Coast that: (1)
reduce or minimize impacts on natural-origin populations…” (1-14)
“…maintain current low levels of hatchery production in order to minimize genetic risks of hatchery fish interbreeding with natural-origin coho salmon, and 2) maintain current low levels of hatchery production in order to minimize competition and predation risks with wild fish in tributaries and estuaries. (1-14 and 15)
“…maintain current low levels of hatchery production in order to minimize competition and predation risks with wild fish in tributaries and estuaries. (1-14)
“…ensure that Indian tribes do not bear a disproportionate burden for the conservation of listed species, so as to avoid or minimize the potential for conflict and confrontation.” (1-17)
“…minimize the area of the stream that may be impacted by a water withdrawal for the hatchery facility. (3-1)
“Although poorly managed hatchery programs can increase disease and pathogen transfer risks, compliance with applicable protocols for fish health can effectively minimize this risk. (3-15)
“…the magnitude of this benefit would be minor because the traps have been managed to minimize delay of fish migration and most of the watershed is not affected by hatchery traps… (4-34)
“…traps have been managed to minimize delay of fish migration..” (4-44)
“Modify or add alternatives to avoid, minimize, or mitigate significant cumulative effects.” (5-2)
“…ESA will ensure that listed species are not jeopardized, and that “take” under the ESA from salmon and steelhead hatchery programs is minimized or avoided.” (5-11)
“…“take” under the ESA from salmon and steelhead fisheries is minimized or avoided. Where needed, reductions in effects on listed salmon and steelhead may occur through changes in areas or timing of fisheries, or changes in types of harvest methods used.” (5-12)
“These plans describe each hatchery program in detail, including fish life stages produced and potential measures to minimize risks of negative impacts that may affect listed coho salmon.”
“Differences between hatchery-origin and natural-origin fish are minimized, and hatchery origin fish are integrated with the local populations included in an ESU or DPS.” (xv)
NMFS Failure To Implement Hatchery Reform
The track record for NMFS’s failure to address hatchery reform and protection of threatened species is well established by its actions in the Columbia River. NMFS has not complied with the ESA and the National Environmental Policy Act (NEPA) when it has funded Mitchell Act hatchery programs and, it has not implemented hatchery reform through the ESA and NEPA. NMFS has failed five times to produce a biological opinion for the federal power systems even though it has the responsibility and authority to implement the ESA and to place effective conditions on hatcheries to protect wild salmonids.
Given this record of noncompliance with the direction of the federal court and failure to implement the ESA to protect endangered salmonids in the Columbia, it is to be expected that hatchery reform through the current NEPA process will also fail to protect threatened coho salmon and other wild salmonid species on the Oregon Coast.
The NEPA DEIS is fatally flawed because it treats hatchery HGMPs as a standalone program. Hatchery impacts on the salmonid ecosystem by moving hatchery fish among watersheds (intentional straying impacts) and straying of hatchery fish that do not return to a hatchery place wild salmonids at risk which the NEPA DEIS fails to quantify. NMFS proposes to “minimize” these impacts but how that is accomplished is not provided. NMFS proposes to control stray rates even though impact on wild salmonids is not provided.
Hatchery salmonids and associated fisheries have genetic and ecological impacts on wild salmonids, but fishery impacts are not quantified even though the stated purpose of the NEPA DEIS is to provide hatchery fish for commercial and sport fisheries. Measurable and enforceable criteria for wild salmonid escapement by watershed and species have not been established.
In addition, the NEPA DEIS fails to establish a habitat management plan that would protect the life history diversity and productivity of Oregon coastal salmonids.
In combination the impacts of hatchery HGMPs and associated fisheries have not been evaluated for impacts
Review and Comments on Trask Hatchery Coho HGMP
Introduction:
I have selected at random two proposed hatchery HGMPs, one for coho salmon and one for steelhead, in order to evaluate the risks and impacts on native naturally spawning salmonids, listed and non-listed under the ESA. The risk factors associated with the HGMPs I have reviewed are likely risk factors associated with all HGMPs considered through this NEPA DEIS. Consequently, all proposed HGMPs would have to be modified to show that native fish (anadromous and resident) are protected before approval by NMFS.
“NMFS believes hatcheries can be managed in a manner that conserves and recovers salmon and steelhead listed under the ESA. Therefore the 4(d) rule provides a way to permit the ‘take’ of listed fish for a variety of hatchery purposes.” (NMFS 2000, page 10) This statement of belief is not consistent with the accumulated weight of peer reviewed scientific research and therefore establishes a bias if not a conflict of interest. Since the NMFS funds hatcheries as well as has authority to recover endangered salmonids protected by the ESA, NMFS may have a dual and conflicting mission. Rather than confine its responsibility to what it believes, NMFS should provide factual scientific information that supports its belief. If there is factual information supporting NMFS’s belief then the permitting process under the 4(d) rule could be appropriately based on science and conflict of interest could be resolved. This would then provide a scientifically sound basis for permitting HGMPs proposed in the NEPA DEIS.
It is an error of evaluation when HGMPs are evaluated in isolation. The purpose of most hatcheries is to provide an economic product for fisheries; hatcheries and harvest are connected in their related impact on the natural ecosystem including wild salmonids and recovery of protected species. In order to confirm the value and solve risks of hatchery\harvest programs must be integrated into a salmonid management plan focused on sustaining productivity and biological diversity wild native fish. Since salmon are locally adapted to their natal rivers each watershed should have measurable criteria to ensure conservation, by species and race, of wild salmonids. The following provides some of the elements for a coherent science based wild salmonid management plan by watershed which need to be considered in any HGMP evaluation.
Establish a Wild Salmonid Management Framework
Premise of Management:
Salmonids are locally adapted and continuing to adapt to the ecological conditions of their natal streams. In addition, populations may express an adaptive structure within watersheds in response to variation and changes in ecological conditions. Management that is a departure from this fact increases risk, causing decline and extinction of native wild salmonids.
Establish an ecological based management framework:
Based on the ecological function of salmonids and watersheds establish an ecological management program for each watershed/subbasin and related migration and estuary habitats that protects and restores genetic and life history diversity of naturally spawning and rearing wild salmonids; establish objectives for abundance, productivity, distribution and diversity for each species and race of native wild fish.
Baselines:
Establish an historic abundance estimate by species and watershed/subbasin.
Establish a genetic and life history diversity benchmark by species and watershed/subbasin.
Identify historic utilization/distribution for each watershed by salmonids to guide reconnecting salmon and habitats.
Habitat and salmonid life history:
Identify and maintain system-wide habitat use by life stage for each species by watershed.
Water quantity and quality is managed to maintain optimum productivity.
Water temperature is maintained within limits that optimize juvenile and adult growth and survival requirements.
Artificial fish passage barriers are removed to provide access to the entire watershed for juvenile and adult salmonids.
Natural barriers are not altered to provide or improve passage of adult salmonids.
In each watershed/subbasin establish a habitat protection and management agreement to support the life history requirements and productivity of wild native salmonids among government agencies, private landowners, counties and towns.
Spawner Abundance Targets:
Establish spawner abundance objectives by species and race for each watershed based on estimate of habitat carrying capacity.
Establish an egg deposition target for each species and watershed for wild salmonids.
Establish nutrient enrichment objectives for each watershed from carcasses of naturally spawning salmonids and achieve them annually.
Harvest Management:
Harvest is regulated to achieve spawner abundance objectives by species and race for each watershed and subbasin. Objectives for spawning timing and size/age of adult spawners are included. Nutrient enrichment of watersheds from salmonid carcasses is achieved. Egg deposition targets by species are achieved. Subbasin fisheries for trout and adults are regulated to support optimum reproduction in each watershed.
Hatchery Management:
Naturally spawning hatchery fish are excluded from wild salmonid spawning areas.
Ecological impacts of hatchery fish on wild fish are identified and controlled.
A stock transfer policy is adopted so that fish and eggs are not transferred among watersheds.
Since hatcheries are used to produce a product for the market economy – commercial and sport fisheries – their purpose is distinctly different from conservation and recovery management for wild salmonids. Integrated evaluation of hatchery\harvest impacts on wild naturally produced salmonids is necessary to define risks and values associated with management for both commodity and conservation purposes. The HGMP protocol as defined (Smith 2006 ) is primarily confined to hatchery operations not their ecological and genetic influence on native wild species once released.
Trask Hatchery Coho Salmon HGMP
(Page 1-3) 1.6) Type of program.
Isolated Harvest – The intent of this propagation program is for harvest augmentation and is not intended to produce fish to spawn in the wild or interbreed with natural populations. This program is currently operated as a segregated hatchery program.
As described within this HGMP, ODFW proposes to establish a new Tillamook Bay basin broodstock using wild Coho Salmon once approved by NOAA. Future production would include some level of integration of wild adults into the broodstock. Issue: The shift to native broodstock is a complete change in the HGMP, directly affecting wild salmon, and should be evaluated in a separate HGMP rather than be treated as an add-on without NMFS review and public comment.
Identified HGMP Risk Issues influencing conservation and recovery of coho salmon:
The following risk factors are identified in the Trask Hatchery HGMP and some were included as risk factors but not identified. The HGMP should evaluate these risks and show how they will be resolved.
(1-3) Estimate of incidental mortality rate on wild Coho Salmon.
(1-5) Temporal distribution of Trask Hatchery adult Coho Salmon returns and broodstock collected.
(1-5) Standard 2.2 Releases of Trask Hatchery Coho smolts will minimize impacts to naturally produced salmonids through control of hatchery release numbers, and by minimizing spatial and temporal overlap with natural populations. When the term “minimize” is used in the HGMP and is not equal to zero impact, the HGMP needs to identify a monitoring plan setting specific criteria to address and resolve risks.
(1-5) Number of Trask Hatchery Coho smolts released will not exceed basin smolt production capacities.
(1-5) Temporal distribution of wild Coho Salmon smolt migration from Tillamook Basin smolt traps (Little North Fork Wilson River EF Trask).
(1-5) Dates of Trask Hatchery Coho smolts releases.
(1-5) Location of Trask Hatchery Coho smolts released.
(1-5) Standard 2.3 Trask Hatchery and Depoe Bay coho smolts will be volitionally released as yearlings. Any fish remaining after the volitional period, will be crowded out of Trask Hatchery. What use volitional release and end up force releasing fish that do not migrate on their own? What is the impact of non-migrant smolts in the natural ecosystem?
(1-5) Beginning and ending dates of Trask Hatchery coho release.
(1-5) Estimated proportion of Trask Hatchery coho leaving volitionally.
(1-5) Standard 2.4 All Trask Hatchery coho smolts will be acclimated and released on station. Any fry or fingerlings in excess of needs for the Trask smolt program may be released into standing water bodies without natural coho production, or may be destroyed.
(1-6) Location of Trask Hatchery Coho smolts acclimation and release.
(1-6) Genetics, Standard 3.1 Hatchery Coho Salmon will not exceed the stray rate identified in the Coastal Coho Conservation Plan (2007) for the Tillamook Basin naturally spawning coho population abundance. The stray rate standard is not stated or justified by scientific criteria. Standards should not be referenced but stated and a link provided to the reference.
(1-6) Estimated abundance of naturally spawning Coho Salmon in Tillamook Basin. Issue: The abundance is estimated not counted.
(1-6) Estimated abundance of naturally spawning Coho Salmon in the Tillamook Basin that are of hatchery origin, based on marks and/or tags. Issue: relies on estimate rather than count
(1-6) Standard 3.2 Stock 34 coho, or wild adult returns from the Tillamook Bay basin, will be used as broodstock. Issue: the shift from segregated to integrated hatchery stock will take place after NMFS approves the HGMP so this shift will not be formally reviewed in the HGMP but needs to be.
(1-6) Location of broodstock collection unknown risk
(1-6) Fin clips on hatchery fish collected for brood, or unmarked adults. Issue: both fin marks and CWT should be used to track strays in Trask and other rivers.
(1-6) Standard 3.3 Stock 34 coho broodstock will be spawned following appropriate mating and spawning protocols to maintain genetic diversity of the population. Issue: protocols need to be stated. Do not ignore scientific information showing captive breeding can change genetic characteristics in one generation.
(1-6) Number and ratio of males and females spawned. Issue: the ratio should be stated.
(1-6) Matings will follow procedures as outlined and appropriate for the stock size, in the Hatchery Management Policy, Fish Health Management Policy, IHOT fish health document, or as directed by the ODFW staff geneticist. Issue: links need to be provided to references.
(1-7) Standard 4.5 Naturally produced steelhead, Chinook Salmon, Coho Salmon, Chum Salmon, and Cutthroat Trout that enter the Trask Hatchery adult traps are handled and released (except fish retained for brood) in a manner that minimizes stress, injury, mortality, and delay in migration. Issue: When the term “minimize” is used in the HGMP and is not equal to zero impact, the HGMP needs to identify monitoring that would be used and what will be done so that minimize is defined using measurable criteria.
(1-7) Number of unmarked adult steelhead, Chinook Salmon, Coho Salmon, Chum Salmon, and Cutthroat Trout collected and released alive (or retained for brood) from the Trask Hatchery traps. Issue: This risk needs to be documented each year and associated mortality evaluated.
(1-7) Number of unmarked adult steelhead, Chinook, coho, chum, and cutthroat mortalities at Trask Hatchery during operation of the hatchery adult traps. Issue: This risk needs to be documented annually. Another issue related to traps is forced spawning below traps by wild salmonids. This impact should also be documented since they fish are prevented from spawning in upstream natal habitat.
(1-7) Standard 4.6 Releases of hatchery coho smolts will limit predation impacts to naturally produced salmonids through control of hatchery release numbers and by minimizing spatial and temporal overlap with naturally produced salmonid juveniles. Issue does not include predator attraction in river and estuary. When the term “minimize” is used in the HGMP and is not equal to zero impact, the HGMP needs to identify monitoring that would be used and what will be done so that minimize is defined using measurable criteria.
(1-7) Dates, location and sizes of Trask Hatchery coho releases. Issue: This risk should be included in research to identify problems and lower risk to a level that protects natural origin salmonids.
(1-7) Temporal and size distribution of wild coho smolt migration from Little North Fork Wilson and EF Trask LCM smolt traps.
5. Socio-Economic Effectiveness
(1-7) Standard 5.1 Estimated harvest benefits will equal or exceed hatchery production costs for Trask Hatchery coho, based on the benefit-cost model in ODFW (1999), or an updated version of that model. Issue: economic benefits and costs to provide those benefits is needed (See Radtke et al. 2016 letter to ODFW and IEAB 2002 Power Council web page. Smolt to adult recruitment for each hatchery release needs to be determined so that the cost to produce salmon that are harvested is determined. This would provide a Benefit\Cost evaluation based on cost to provide a harvest benefit which is the stated purpose of the hatchery program.
(1-8) 1.11.1 ODFW proposes to establish a new program using wild coho adults. A maximum of 110 adults (55 females and 55 males) would be needed for broodstock to meet full production goals. Issue: impact on wild spawner population genetic change and interbreeding, straying and homogenizing wild coho by hatchery program. Research should be required to evaluate impact to wild coho and measureable criteria developed to reduce those impacts that protect wild coho and contribute to recovery.
(1-8) 1.12 Catch estimate represents landed catch and does not include an estimate of non-landed coho mortality in chinook only fisheries. Issue: mortality of wild coho is not fully estimated including wild release encounters needed to harvest a hatchery fish in ocean and river fisheries. Research should be conducted to determine the level of risk and recommend methods to reduce it.
(1-9) average 9% hatchery fish on the spawning grounds (Range 3% to 14%). Therefore the existing program design of 100,000 smolt releases appears to be in compliance with the Native Fish Conservation Policy and the Coastal Coho Conservation Plan stray rate limits. Issue compliance with NFCP needs to be determined and ability to control excessive stray rates needs to be tested. Using averages is not justified. This information would be provided annually.
(1-11) 1.15 Watersheds targeted by hatchery program: The Trask River Basin within the Tillamook Bay Basin is the release site and desired return site for Trask Hatchery coho released on station. Hatchery coho from these releases are expected to return to the Trask Hatchery site. Issue: evaluation of stray hatchery coho is needed and reported annually.
The North Fork Depoe Creek Basin within the Depoe Bay Basin is the release site and desired return site for all Depoe Bay STEP coho released under this program. Coho Salmon from these releases are expected to return to the NF Depoe Bay Creek site. Issue evaluation of stray hatchery coho is needed based on measurable criteria and reported annually.
(1-11) 1.12 Alternative 1 Increase Releases of Stock 34 : impacts from stock-34 hatchery coho on naturally rearing species would potentially increase. Issue: The costs and potential benefits are noted but the cost to determine the impact of increased interbreeding of hatchery (stock 34) on wild coho is not mentioned. It should be added and this alternative would have to develop a monitoring and evaluation plan along with funding support. The purpose of this plan is to determine the risk to ESA listed coho salmon and compliance with NMFS PVA criteria.
(1-11) Alternative 4 Incorporate wild coho into hatchery broodstock: Use of unmarked, wild adults would replace the current broodstock. Alternatively, a wild brood program could be phased in during years of sufficient abundance while maintaining a portion of the releases from the existing program. Use of naturally produced adults from the Tillamook Bay basin may increase potential straying of returning adults within the basin. This alternative is being proposed in this HGMP for implementation. Issue: Low performance of domesticated hatchery coho would be replaced with wild coho broodstock to increase contribution to fisheries at the risk of increased strays and interbreeding with wild coho. A monitoring and evaluation plan recommended for Alternative I would apply here as well.
(1-12) Alternative 5 Install a hard weir at Trask Hatchery: Issue The description is negative and does not include passage options already in use that require no handling of fish at Warm Springs Hatchery and the boat slip at a weir on the NF Santiam.
(1-12) Alternative 6 – Develop a new broodstock from wild, naturally produced fish from the Trask River. Description and Implications - It is unknown if sufficient numbers of naturally produced fish would be available to meet genetic needs without excessive impact to natural populations in the Trask basin. The consumptive coho sport fishery in the Tillamook basin would be maintained. Interactions of hatchery coho with naturally produced fish may result in negative impacts to wild coho populations. Issue: This includes risks associated with mining wild coho for eggs, genetic and ecological impacts on wild coho, and replacement of wild coho with hatchery product. The monitoring and evaluation plan recommended for Alternative 1 would apply here too.
(2-1) Tillamook coho complex: There is an estimated 250 miles of spawning habitat available to the Coho Salmon of this complex. Issue: According to research on marine derived nutrient derived for coho salmon of 120 carcasses per km (Bilby et al. 2001). Using this recommendation would mean the spawner goal for the Tillamook coho complex is 48,280 spawners to achieve needed nutrient enrichment from coho salmon carcasses.
The DEIS estimates that “The critical population level of Coho Salmon for the Tillamook Complex is 1,000 adult spawners. However, this complex is not considered to be viable because high-quality habitat is estimated to be present in only 12 miles of stream, below the 15-mile threshold needed to support a viable population.” (2.2.2) Status of ESA-listed salmonid population(s) affected by the program.) Using the Bilby et al. 2001 formula for minimum coho carcasses the spawner level should be 2,400 coho spawners. This is more than twice the minimum adult spawner estimate in the DEIS. Issue: A better approach would be to use the Bilby formula for wild coho spawner escapement, increase nutrient enrichment, invest in restoring additional three miles of high-quality habitat, manage fisheries to secure needed escapement of wild coho as a recovery action to increase the minimum critical population level.
The Tillamook historically was one of the most productive salmon areas on the Oregon coast. “The abundance of wild Coho Salmon spawners in the Tillamook Complex has ranged from about 1,300 to 20,000 and has averaged about 8,500 since 2003” (table 2.2). The Tillamook Complex is now producing, on average, more wild coho salmon than estimated for high-quality habitat. This would suggest that the estimate of high quality habitat is low or a combination of high and low quality habitat is more productive than recognized. A well-managed wild coho population would set the intrinsic productivity for the Tillamook Complex at more than 20,000 spawners. This would be even higher when spawners removed by the fishery are included in the estimated intrinsic productivity. The Tillamook Complex should not be managed for minimum escapement (critical population level) but for its intrinsic productivity and for the nutrient enrichment target recommended by Bilby (2001).
Truncated Data Contributes to Shifting Baselines:
Another problem with Table 2.2 is the limited years of data (2003-2015). The ODFW has collected historic estimates for coho salmon, an important commercial species, since 1892 (Mullan 1981) Confining the data on wild coho from 2003- 2015 promotes the problem of shifting baselines. According to Mullan (1981) the “In-basin commercial coho harvest” for Tillamook Bay ranged from a low of 23,000 fish to 70,000 fish. The average catch from 1923 to 1940 was 43.6 thousand fish. In 1923 a poundage fee was imposed and the data quality improved. In 1940 the bay was closed to commercial harvest. During this time the Tillamook Complex salmon habitat was damaged by splash dams, logging and forest fires, so the habitat was likely of lower productivity than it is today. This may mean that the Tillamook Complex is more productive than recognized and management should “…ensure that escapements are sufficient to perpetuate maximum biomass production and biodiversity (Knudsen 2000).
Knudsen (2000) also says, “We cannot count on repairing only one damaged aspect of salmon runs (e.g. degraded habitat) to fix the problem, but must work on all fronts simultaneously. Ultimately, though, both productivity and biodiversity depend on sufficient escapement of spawners to fully utilize the available freshwater habitat, fertilize the systems with carcasses, and optimize genetic diversity.”
There is absolutely no justification by ODFW to overlook this and other relevant scientific evaluation data in constructing its HGMPs protocols.
(2-6) No data is available for progeny of naturally spawning hatchery coho rearing in the wild.
(2.7) The HGMP states: The potential for take of a listed stock during this trapping is low and would consist of migrational delay, capture, handling, and release during the operational period. Trapping and handling devices and methods may lead to injury of listed fish through descaling, delayed migration timing and spawning, and delayed mortality as a result of injury or increased susceptibility to predation. Any unmarked fish collected during this trapping, if not retained for brood, are immediately released back into Gold Creek or Trask River. Any take would be incidental take.
Comment: Using weirs can also result in displaced spawning. That is, the wild fish held below a weir may spawn and in doing so the eggs and juveniles may be in less suitable habitat and can be more vulnerable to mortality. Fish spawning below a weir is not spawning in natal habitats and are likely to have low reproductive success. This problem has been identified on the Sandy River where wild spring chinook are spawning below weirs rather than reaching their natal spawning areas.
The take may be defined as incidental, yet it is a purpose of management to protect wild threatened coho salmon. Leaving it as an incidental take without an evaluation of potential or real impact on a threatened species is an inappropriate response in the HGMP that is designed to control risk to wild coho and support recovery. The impact should be documented in annual operation reports numerically, and mitigation actions to address impacts stated. There should be an evaluation as to the competence of the mitigation action for protection of wild coho.
Summary for risk factors identified in the HGMP:
To be complete the HGMP should include a section that addresses how the identified risks are to be evaluated, monitored and resolved including the funding available to accomplish required work. In the Oregon Coastal Multi-Species Plan (CMP) there are numerous statements by ODFW about funding being a limiting factor on what actions are actually carried out. Therefore funding limitations related to resolving risks identified in the HGMP must be available so it is possible to determine which risks will be addressed and resolved. This information should be included in a requirement for an annual report documenting actions taken to address risks showing which risks were resolved and those that remain to be resolved. This information is not typically found in annual hatchery operation plans. The lack of funding to resolve risks is not an acceptable excuse and would mean the HGMP is not functioning as agreed to with NMFS. NMFS should develop a response to address the failure of the HGMP. NMFS and the public would then have an annual update about risks resolution or failure to do so. This reporting should be required for all HGMPs proposed by ODFW for the Oregon Coast.
Siletz Winter Steelhead HGMP Concerns:
- The program takes wild steelhead to propagate its hatchery fish via a brood-stock program with an allowance of taking up to ½ of the encountered wild winter steelhead, but no more than 40 pairs, at the Siletz falls trap for this purpose, effectively removing them from spawning in the wild. The actual population size of Siletz winter steelhead is currently unknown. The overall goal of the program is to take no more than 10% of the overall wild winter steelhead population. (Section 1.5/1.7/1.9/1.10/1.11/6.2.2/7.4.1). The goal for taking wild brood stock without knowing the run size is a risk to the wild population.
- Release of 50,000 smolts creates competition for limited resources with wild smolts as they migrate to the ocean (Section 1.7). Potential for increasing predator attraction that affect wild salmonids in the river and estuary.
- Stray rates are greater than 10% as outlined in Standard 4, Impacts to wild fish: “Limit hatchery fish to 10 percent or less of the fish spawning in natural habitats of the Siletz and neighboring basins, except in the immediate area (within 1 mile) around the release site(s).” The HGMP provides that, “Estimates of the total number of hatchery winter steelhead that strayed to all natural spawning areas in the Siletz Basin are not available. Therefore, data for the “Spawning Grounds” column (Table 1-2) is not available. Data on stray rates is available for three trap sites within the Siletz Basin that are removed from the smolt release site. These traps are on Schooner Creek, Mill Creek and Siletz Falls. The average hatchery stray rate (all stocks of hatchery fish) seen at these traps has ranged from 45 to 93 percent (1998- 2007). The stray rate for just the stock 33W from 1998 through 2007 has ranged from 40 to 70 percent at Mill Creek, and from 34 to 65 percent at Siletz Falls. Both of these sites are removed from the release site, although the Siletz Falls site is on the mainstem and may not represent levels seen in tributaries. The stock 33W has been seen in five different years (1998-2007 ) ranging 1-5 fish in the Schooner Creek trap; the only other site in the Siletz Basin used as a monitoring site. Very few fish of this stock have been recorded in sites outside of the Siletz Basin, which includes several adult traps in neighboring basins.” “A key issue related to this hatchery program is the overall abundance of hatchery steelhead spawning in natural habitats occupied by wild winter steelhead in the Siletz Basin. Observations in tributaries of the Siletz River indicate a substantial proportion of hatchery spawners in winter steelhead habitats. Some of these hatchery spawners are thought to be Siletz hatchery winter steelhead. These fish have an adipose and left maxillary fin clip. However, other hatchery programs in near by coastal basins also release winter steelhead with the same mark so it is uncertain to what extent hatchery Siletz winter steelhead stray. There is also concern that large numbers of hatchery steelhead smolts released in the Siletz Basin may create competition with wild fish or attract predators which could also affect wild fish.” (Section 1.9/1.10/1.12/1.16)
- The HGMP assumes that there are no genotypic or phenotypic differences between brood stock and wild fish so pose no threat to the wild population (Section 6.2.4).
- It is possible that by selecting wild fish at the Siletz falls for the brood stock program there is an increased propensity for the hatchery progeny to stray as those fish encountered at the falls are straying from the lower river as wild winter steelhead are not passed above the falls (Section 7.2)
Summary:
Our comments address risks to ESA listed coho salmon and to candidate steelhead along other native, wild salmonids in Oregon coastal rivers relative to the DEIS. This means that the hatchery HGMPs fail to protect ESA listed coho and will contribute to the take of this species. The HGMPs are not being operated or proposed to be operated consistent with the best available science regarding genetic and ecological impacts on wild salmonids. We have evaluated the alternatives provided in the DEIS and show that they are not adequate to protect threatened coho and other species. The NMFS needs to conduct an evaluation of the Oregon coastal hatchery programs with full involvement of the Protected Species Program and the Science Center that would establish measurable criteria for operation of each hatchery program HGMP on the environment.
Sincerely,
Bill Bakke, Founder and Director of Conservation and Science
References:
Araki, Hitoshi, Barry A. Berejikian, Michael J. Ford, and Michael S. Blouin. 2008. Fitness of hatchery-reared salmonids in the wild. Blackwell Publishing Ltd. 1:342-355.
Araki, Hitoshi, Becky Cooper, and Michael S. Blouin. 2009. Carry-over effects of captive breeding reduces reproductive fitness of wild-born descendants in the wild. Biological Letters 5: (5) 621-624.
Araki, H., and C. Schmid. 2010. Is hatchery stocking a help or harm?: Evidence, limitations and future directions in ecological and genetic surveys. Aquaculture 308:S2-S11.
Bilby, Robert E., Brian R. Fransen, Jason K. Walter, C. Jeff Cederholm, and Warren J. Scarlett. 2001. Preliminary Evaluation of the Use of Nitrogen Stable Isotope Ratios to Establish Escapement Levels for Pacific Salmon. American Fisheries Society. Fisheries Vol. 26 No. 1.
Buhle, E. R., K. K. Holsman, M. D. Scheuerell, and A. Albaugh. 2009. Using an unplanned experiment to evaluate the effects of hatcheries and environmental variation on threatened populations of wild salmon. Biological Conservation 142:2449-2455.
Chilcote, M.W., K.W. Goodson, and M.R. Falcy. 2013. Corrigendum: Reduced recruitment performance in natural populations of anadromous salmonids associated with hatchery-reared fish. Can. J. Fish. Aquat. Sci. 70: 1-3.
Christie, Mark R., Michael J. Ford, and Michael S. Blouin. 2014. On the reproductive success of early-generation hatchery fish in the wild. Evolutionary Applications. John Wiley and Sons Ltd.
Christie, Mark R., Melanie L. Marine, Samuel E. Fox, Rod A. French & Michael S. Blouin. 2016. A single generation of domestication heritably alters the expression of hundreds of genes. Nature Communications.
Dittman AH, Quinn TP (1996) Homing in Pacific salmon: mechanisms and ecological basis. J Exp Biol 199:83–91
Ford, Michael J,. 2002. Selection in Captivity during Supportive Breeding May
Reduce Fitness in the Wild. Conservation Biology, Volume 16, No. 3: 815–825
Grant (editor). 1997. Genetic effects of straying of non-native hatchery fish into natural populations. Proceedings: of the workshop. U.S. Dep. Commer., NOAA Tech Memo NMFS-NWFSC-30, 130 p.
ISAB\ISRP. 2016-1. Critical Uncertainties for the Columbia River Basin Fish and Wildlife Program. N.W. Power Planning and Conservation Council.
Keefer, Matthew L. and Christopher C. Caudill. 2014. Homing and straying by anadromous salmonids: a review of mechanisms and rates. Fish Biol. Fisheries 24:333–368
Kostow, Kathryn, Anne Marshall, and Stevan R. Phelps. 2003. Naturally spawning hatchery steelhead contribution to smolt production but experience low reproductive success. Trans. Am. Fish. Soc. 132:780-790.
Knudsen, Eric, E. 2000. Sustainable Fisheries Management: Pacific Salmon. Eds. E. Eric Knudsen, Cleveland R. Steward, Donald D. McDonald, Jack E. Williams, and Dudly W. Reiser. Lewis publishers.
Lynch, M., and M. O’Hely. 2001. Captive breeding and the genetic fitness of natural populations. Conservation Genetics 2:363–378.
McLean, Jennifer E., Paul Bentzen, and Thomas P. Quinn 2004. Differential reproductive success of sympatric naturally spawning hatchery and wild steelhead trout through the adult stage. Can. J. Fish. Aquat. Sci. 60(4): 433-440.
Marchetti MP, Nevitt GA (2003) Effects of hatchery rearing on brain structures of rainbow trout, ncorhynchus mykiss. Environ Biol Fish 66:9–14
McCormick SD, O’Dea MF, Moeckel AM, Bjørnsson BT (2003) Endocrine and physiological changes in Atlantic salmon smolts following hatchery release. Aquaculture 222:45–57
McElhany, P., M.H. Ruckelshaus, M.J. Ford, T.C. Wainwright, and E.P. Bjorkstedt. 2000. Viable salmonid populations and the recovery of evolutionarily significant units. U.S. Dept. Commer., NOAA Tech. Memo. NMFS-NWFSC-42,156 p.
McMichael, Geoffrey A., Cameron S. Sharp, and Todd N. Pearsons. 1997. Effects of residual hatchery reared steelhead on growth of wild rainbow trout and spring chinook salmon. Transactions of the American Fisheries Society 126: 230-239.
McMichael, Geoffrey A., Todd N. Pearsons, and Steven A. Leider. 1999. Behavioral Interactions among Hatchery-Reared Steelhead Smoltsand WildOncorhynchus mykiss in Natural Streams. North American Journal of Fisheries Management 1999; 19: 948-956
Naish, K. A., J. E. Taylor, P. S. Levin, T. P. Quinn, J. R. Winton, J. Huppert, and R. Hilborn. 2008. An evaluation of the effects of conservation and fishery enhancement hatcheries on wild populations of salmon. Advances in Marine Biology 53:61–194.
Nickelson, Tom. 2003. The influence of hatchery coho salmon on the productivity of wild coho salmon populations in Oregon coastal basins. Can. J. Fish. Aquat. Sci. 60: 1050-1056.
NMFS June 20, 2000 The Citizen’s Guide to the 4(d) Rule for Threatened Salmon and Steelhead on the West Coast, page 10. http://www.westcoast.fisheries.noaa.gov/publications/fishery_management/salmon_steelhead/2016_final_complete_salmon_ea_648-bf56.pdf
NMFS 2008. Biological Recovery Criteria for the Oregon Coast Coho Salmon Evolutionarily Significant Unit
NOAA Technical Memorandum NMFS-NWFSC-91. May 2008. http://www.dfw.state.or.us/fish/crp/docs/coastal_coho/reference/BRCohoFinal.pdf
ODFW 2014 Coastal Multi-species Conservation and Management Plan
Ogston, Lindsey, Sam Gidora, Matthew Foy, and Jordan Rosenfeld. 2015. Watershed scale effectiveness of floodplain habitat restoration for juvenile coho salmon in the Chilliwack River, British Columbia. Can. J. Fish. Aquat. Sci. 72: 479-490.
Oosterhout, Gretchen R., Charles W. Huntington, Thomas E. Nickelson, and Peter W. Lawson. 2005. Potential benefits of a conservation hatchery program for supplementing Oregon coast coho salmon (Oncorhynchus kisutch) populations: a stochastic model investigation. Can. J. Fish. Aquat. Sci. 62: 1920–1935.
Paquet, P. J., T. Flagg , A. Appleby , J. Barr , L. Blankenship , D. Campton , M. Delarm , T. Evelyn , D. Fast , J. Gislason , P. Kline , D. Maynard , L. Mobrand , G. Nandor , P. Seidel & S. Smith (2011) Hatcheries, Conservation, and Sustainable Fisheries—Achieving Multiple Goals: Results of the Hatchery Scientific Review Group's Columbia River Basin Review, Fisheries, 36:11, 547-561, DOI: 10.1080/03632415.2011.626661
Reisenbichler, R. R. & McIntyre, J. D. 1977 Genetic differences in growth and survival of juvenile hatchery and wild steelhead trout, Salmo gairdneri. Can. J. Fish. Res. Board 34, 123–128.
Reisenbichler, R.R. and S. P. Rubin. 1999. Genetic changes from artificial propagation of Pacific salmon affect the productivity and viability of supplemented populations. ICES Journal of Marine Science, 56: 459-466.
Scheuerell, Mark D., Eric R. Buhle, Brice X. Semmens, Michael J. Ford, Tom Cooney, and Richard W. Carmichael 2015. Analyzing large-scale conservation interventions with Bayesian hierarchical models: a case study of supplementing threatened Pacific salmon. Ecology and Evolution published by John Wiley & Sons Ltd.
Schroeder, Kirk R., Robert B. Lindsay & Ken R. Kenaston. 2001. Origin and Straying of Hatchery Winter Steelhead in Oregon Coastal Rivers. Transactions of the American Fisheries Society. Volume 130, Issue 3. 431-441
Seamons, Todd R., Lorenz Hauser, Kerry A. Naish and Thomas P. Quinn. 2012. Can interbreeding of wild and artificially propagated animals be prevented by using broodstock selected for a divergent life history? Evolutionary Applications.
Smith, Stephen.2006. Hatchery and Genetic Management Plan (HGMP) Template: Purpose, Applications, and Instructions. NMFS Memorandum: January 5, 2006.
Theriault, Veronique, Gregory R. Moyer, Laura S. Jackson, Michael S. Blouin and Michael Banks. 2011. Reduced reproductive success of hatchery coho salmon in the wild: insights into most likely mechanisms. Molecular Ecology. Blackwell Publishing Ltd.
Utter, Fred. 1998. Genetic problems of hatchery-reared progeny released into the wild, and how to deal with them. Bulletin of Marine Science, 62(2): 623–640.